40 why are metals good conductors of heat and electricity
Is Wood A Better Conductor Of Heat Than Plastic - PixAria Complete answer: -Good conductors are those which can conduct heat and electricity. Example of a bad conductor of heat is wood plastics. What are the bad conductors of heat? Metals and stone are considered good conductors since they can speedily transfer heat, whereas materials like wood, paper, air, and cloth are poor conductors of heat. Why are some metals are comparatively good conductors of ... Metals are good conductors of heat and electricity because there is a high concentration of free electrons in a metal. Free electrons, those not bound to individual atoms, are able to transport energy from one part of the metal to another. That is the basis of heat conduction (heat is energy). Glass has no free electrons.
Why Are Metals Good Conductors Of Electricity And Heat? The majority of materials that conduct heat and electricity are metals, for the simple reason that metals contain a glut of free electrons. What Are Free Electrons?

Why are metals good conductors of heat and electricity
Why are metals good conductor of heat and electricity. A(n)____is a meterial that does not permit the flow of heat.* 1 point A: Insulator, Conductor B: Conductor, Electric Field C: Insulator, Electric Field D: Conductor, Chemistry An element that is malleable, ductile, and a good conductor of heat and electricity would be classified as a a)metalliod b)semimetal c)metal d)nonmetal Why Are Metals Such Good Conductors of Heat? Metals conduct heat well for two reasons: metal ions pack very closely together in their molecular lattice, and electrons drifting through the metal carry kinetic energy around the lattice. The result is a quick elevation in particle motion that is expressed through heat energy. Why do metals conduct heat and electricity so well? Metal is a good conduction of heat. Conduction occurs when a substance is heated, particles will gain more energy, and vibrate more. These molecules then bump into nearby particles and transfer some of their energy to them. This then continues and passes the energy from the hot end down to the colder end of the substance.
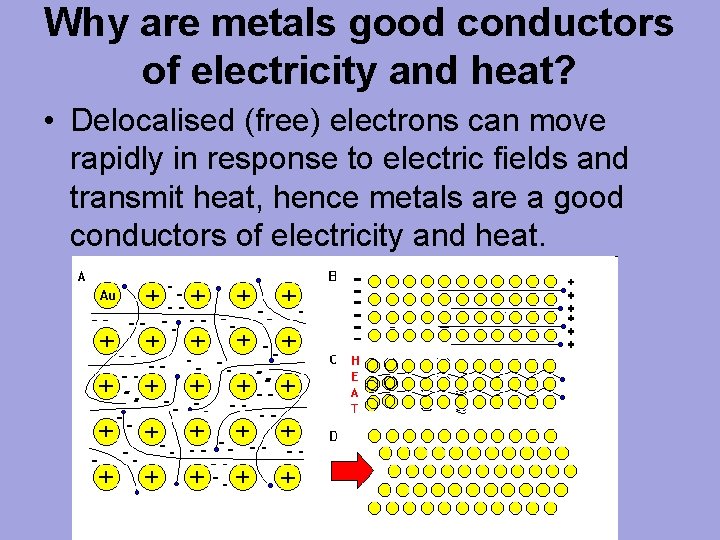
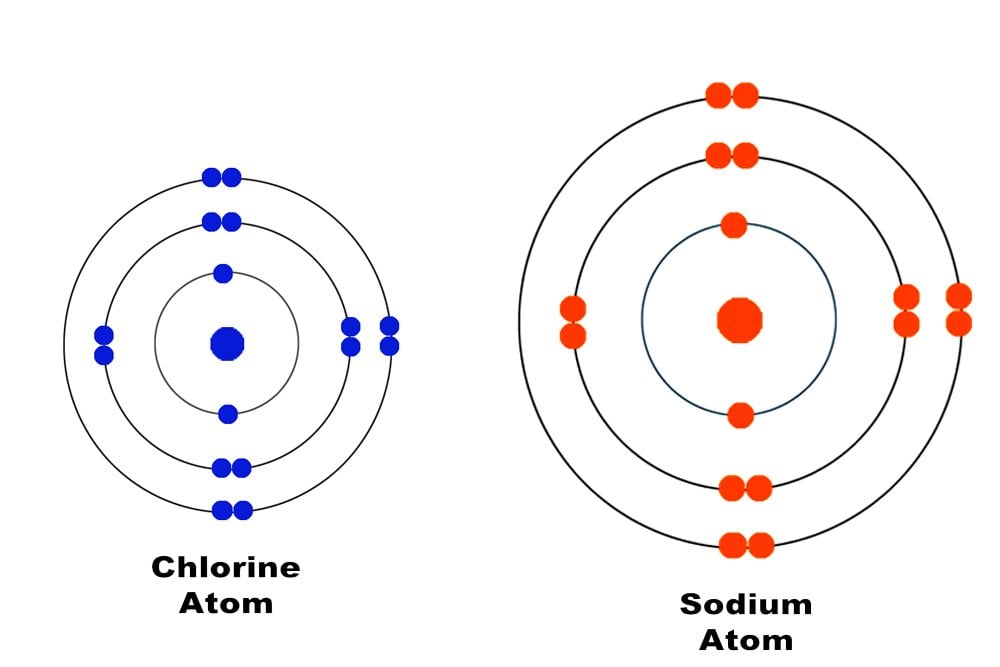
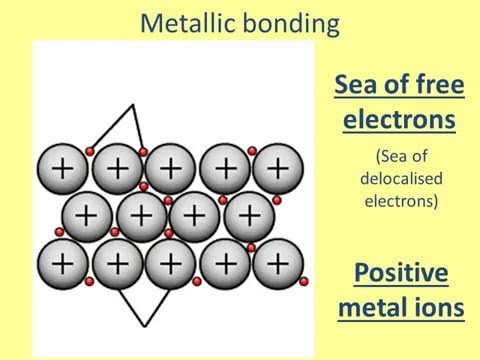
Why are metals good conductors of heat and electricity. What are 5 good conductors of heat? - R4 DN Metals are an excellent conductor of electricity and heat because the atoms in the metals form a matrix through which outer electrons can move freely. Instead of orbiting their respective atoms, they form a sea of electrons that surround the positive nuclei of the interacting metal ions. What are electronic conductors? Why Are Metals Good Conductors of Electricity? Łukasz Klepaczewski/Pixabay Metals are good conductors of electricity because of their atomic structure that allows electric charges to pass through freely. The atomic structure of metals is unique because of the presence of one, two or three valence electrons in the outer orbit of the atoms, and this is true for all metallic elements. Physics Ch. 22 Flashcards - Quizlet Why are metals good conductors of both heat and electricity? The outer shell electrons in metals are free to move from atom to atom. "loose" outer shell e-Why are materials such as glass and rubber good insulators? Electrons are tightly bound to their atoms, making them poor conductors of heat. Why Metals are good conductors of electricity and heat ... Metals form giant structures in which electrons in the outer shells of the metal atoms are free to move. The metallic bond is the force of attraction between these free electrons and metal ions. Metals are good conductors of electricity and heat. This is because the free electrons can move throughout the metal.
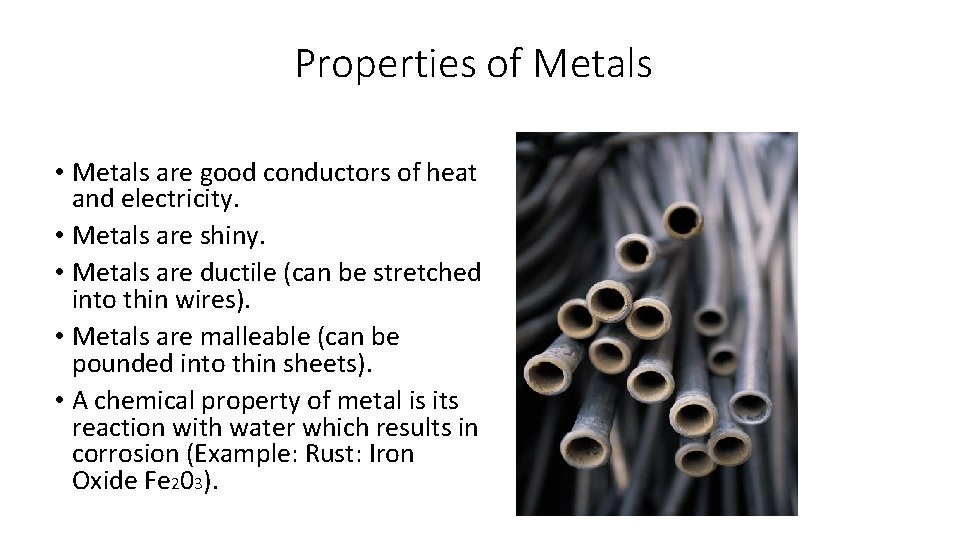
Why are metals good conductors of heat and electricity ... Metal particles are held together by strong metallic bonds, which is why they have high melting and boiling points. The free electrons in metals can move through the metal, allowing metals to conduct electricity. Metal is a good conductor because it absorbs heat easily. Whether hot or cold. Click to see full answer Why Do Metal Conduct Electricity? Explained by Experts Metal - It is a kind of material when freshly prepared, polished, or fractured is a good conductor of heat and electricity. At room temperature, metal tends to be solid with the characteristics of lusture. Also, the metals are malleable and ductile. (These both terms mean the malleable can be harmed by the sheets and the ductile drawn into wire). Why are metals good conductors of both heat and electricity Answer. REASON : Metals are good conductors of electricity, because they have free electrons. These free electrons act as charge carriers in the metallic structure, allowing electric current to flow through the metal. option : 3. Widget. 04:59. Why Are Metals Good Conductors Of Electricity ... So, metals are good conductors of electricity because metals have free electrons. Why metals are good conductors of electricity and heat? Metals are good conductors (both of heat and electricity) because at least one electron per atom is free: i.e., it is not tied to any particular atom, but is, instead, able to move freely throughout the metal.
Why are good conductors of heat also good ... - Quora For example metal are good conductors because there electrics are free to move and there atomic bonding in not very strong. While polymers and ceramics are poor conductors of heat because their fight covalent and ionic bonds are stronger and electrons are not free to move . Why do metals conduct electricity and heat? - Quick Answer Why metals are good conductors of electricity and heat. The reason why metals are good conductors has to do with the nature of their electrons. The outer, or valence, electrons in metals are shared by all the atoms. We call these electrons "delocalised" as they are not associated with a single atom or bond. Below their melting points ... Why is it important to know the properties of metals? By the way, metals have important characteristics.Conductivity :- By saying that metals are good conductors of heat/electricity we mean that metals allow heat/electricity to pass through them easily. Malleability :- The property which allows the metals to be hammered into the thin sheets is called malleability. Why are metals good conductors of both heat and electricity Why are metals good conductors of both heat and electricity? The outer shall electrons in metals move with zero resistance, making all metals superconductors. The outer shell electrons in metals are tightly bound, making it easy for vibrations to move from one atom to the next.
Why are good conductors of heat good conductors of ... Accordingly, why Metals are good conductors of electricity and heat? Metals are particularly good conductors of heat because their particles are very closely packed so the vibrations are passed on very quickly. They also contain large numbers of free electrons.
The Reasons Why Metals Are Such Good Conductors - Engineer Fix What Metals are the Best Conductors For a material to become a good conductor it must allow the electricity that flows through it to move the electrons. The more free electrons in a material, the greater the conductivity of the material. Silver Silver Silver is known as the most conductive metal as it contains a higher number of movable atoms.
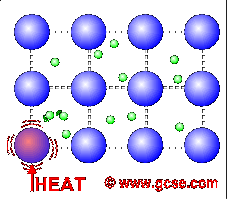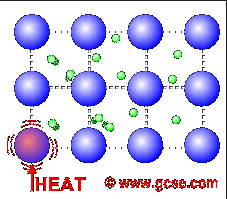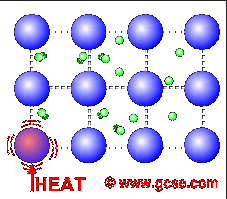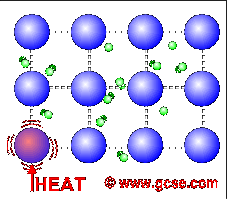
Why is metal a good conductor of heat and electricity ... Metals are good conductors of heat. There are two reasons for this: the close packing of the metal ions in the lattice. the delocalised electrons can carry kinetic energy through the lattice. Why does the ability of a metal to conduct electricity decrease with increasing temperature?
Why Are Metals Good Conductors Of Both Heat And Electricity? The outer electrons in the atoms will vibrate in place and emit electromagnetic radiation that travels throughout the metal. answer Reason : Metals are good conductors of electricity because they contain free electrons. These free electrons act as charge carriers in the metallic structure, allowing an electric current to flow through the metal.
why are transition metals good conductors of heat and ... Why Are Metals Good Conductors Of Heat And Electricity? They are electrical conductors because their delocalised electrons carry electrical charge through the metal. They are good conductors of heat and electricity The compounds of transition metals are usually brightly colored and are often used to color paints.
What Materials Are Good Conductors Of Heat And Electricity ... Answer: Materials that allow heat to pass through them easily are called good conductors of heat. Metals like copper and aluminium have the highest thermal conductivity while steel and bronze have the lowest. Gold, Silver, Iron etc are also some examples of good heat conductors as well as electrical conductors.
Why are Metals Good Conductors of Electricity? - Science ... Heat get transmitted easily through metals by the means of these free electrons. When heat is applied to the metal, the free electrons near the source of heat gain a lot of energy, and start moving rapidly. As the metal has a close packed structure, the energized free electrons collide with other nearby electrons.
Why are good conductors of heat good conductors of ... Furthermore, why Metals are good conductors of electrical energy and warmth? Metals are notably good conductors of warmth as a result of their particles are very intently packed so the vibrations are handed on in a short time. Additionally they include massive numbers of free electrons.
Materials that are good conductors of heat and electricity ... Which among the materials are good conductors of heat and electricity? answer choices. paper and paper clip. cooking pots and pans. copper wire and pencil. metal spoon and towel. paper and paper clip. alternatives. cooking pots and pans.
Metals are good conductors of heat and electricity. Why? Same for electricity , when potential differences (PD) is applied accrues end of a metallic conductor ,they start moving from negative end to positive end of the conductor giving rise to flow of current which is from positive to negative end. Because of that metal is good conductor of heat and electricity.
Why do metals conduct heat and electricity so well? Metal is a good conduction of heat. Conduction occurs when a substance is heated, particles will gain more energy, and vibrate more. These molecules then bump into nearby particles and transfer some of their energy to them. This then continues and passes the energy from the hot end down to the colder end of the substance.
Why Are Metals Such Good Conductors of Heat? Metals conduct heat well for two reasons: metal ions pack very closely together in their molecular lattice, and electrons drifting through the metal carry kinetic energy around the lattice. The result is a quick elevation in particle motion that is expressed through heat energy.
Why are metals good conductor of heat and electricity. A(n)____is a meterial that does not permit the flow of heat.* 1 point A: Insulator, Conductor B: Conductor, Electric Field C: Insulator, Electric Field D: Conductor, Chemistry An element that is malleable, ductile, and a good conductor of heat and electricity would be classified as a a)metalliod b)semimetal c)metal d)nonmetal











/the-most-conductive-element-606683_FINAL-cb8d31a0404241e2a3187e67c7b57e8c.gif)











.png)

![4.5 Why are Metals Malleable and Good Conductors? [SL IB Chemistry]](https://i.ytimg.com/vi/UfovGpgRNx8/maxresdefault.jpg)


0 Response to "40 why are metals good conductors of heat and electricity"
Post a Comment