43 alka seltzer temperature experiment
Alka Seltzer Tablet Experiment Lab Report Lab Report Experiment Seltzer Tablet Alka. The active ingredients in an Alka-Seltzer tablet is aspirin, also known as acetyl-salicylic acid (C8H12O4), citric acid (C6H8O7), and sodium bicarbonate (NaHCO3)2 Gre Practice Essay Topics The experiment involved using Alka-Seltzer antacid tablets, water and hydrochloric acid at different temperatures. Second, I poured some glucose into the beaker and ... How Fast Does Alka Seltzer Make Gas | ipl.org The topic of research is, "how fast does an Alka-Seltzer tablet make gas?". In the experiment, the scientists will be measuring the chemical reaction rates that occur, when 1 Alka-Seltzer tablet is placed in a specific temperature of water. The independent variable during the experiment will be the temperature of the water (degrees Celsius).
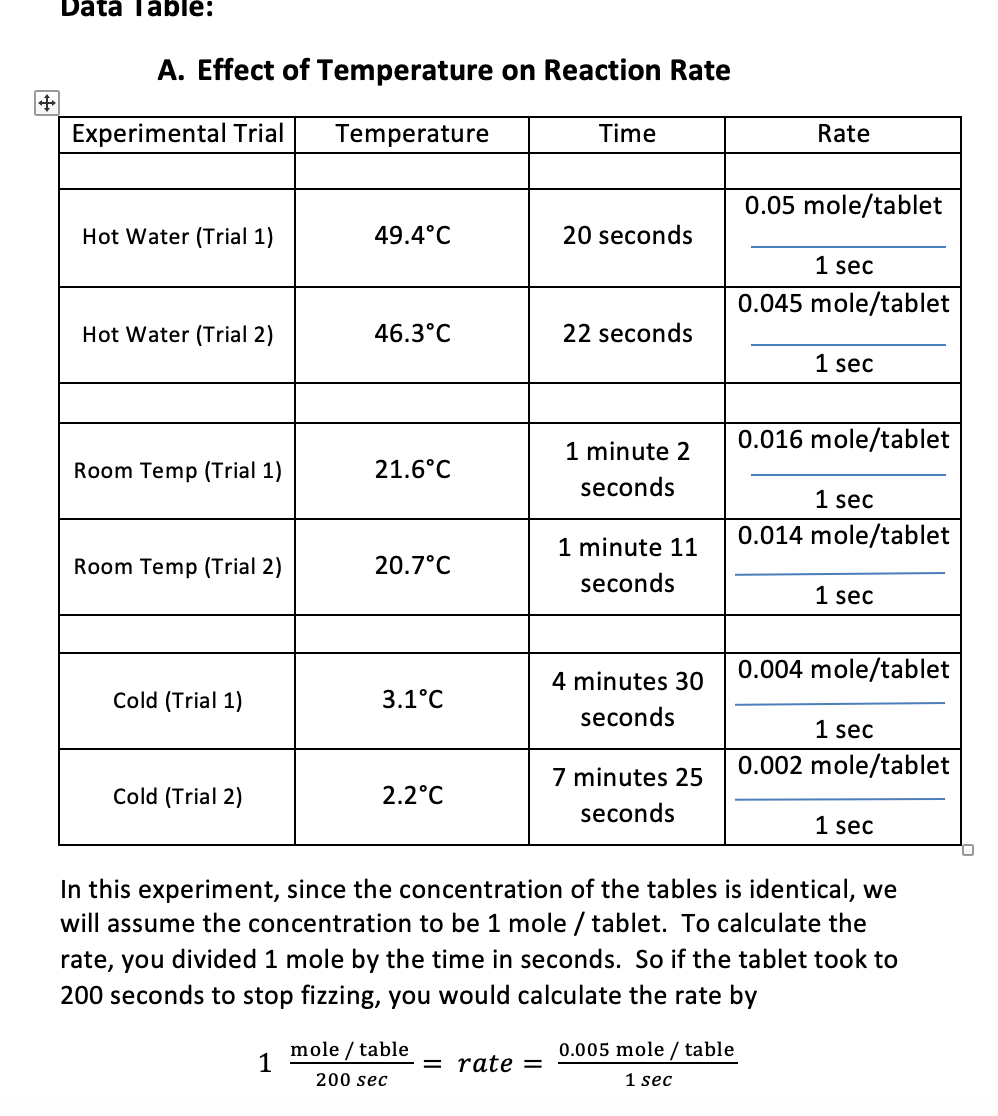
PDF Temperature & Chemical Reaction Rate Lab 3 Alka-Seltzer tablets (Each tablet broken into fourths) Timer . Mortar and pestle . Water - Cold, Hot, Room Temperature. Procedures: A. Hot Water 1. Fill a beaker with 250ml of hot water. 2. Use the thermometer to take the temperature and record it on your data sheet. 3. Drop a quarter of an Alka -Seltzer tablet into the hot water. 4.

Alka seltzer temperature experiment
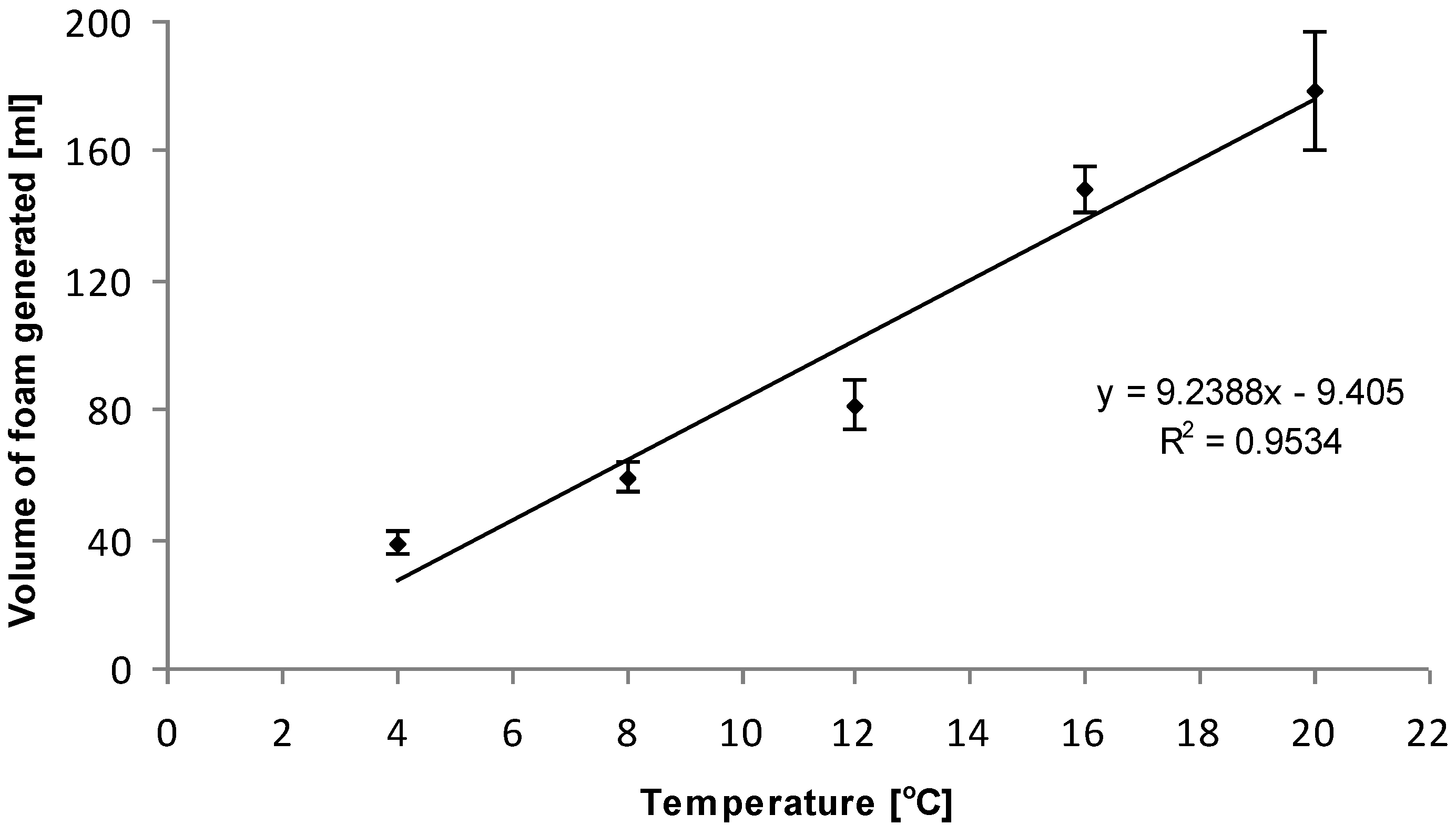
Alka-Seltzer Science: The Effect of Temperature on Reaction Time | STEM Activity Have you ever wondered why bubbles form when an Alka-Seltzer tablet is put in water? If you’ve ever tried it, you’ve seen that the tablet fizzes furiously when dropped into water. The moment the tablet starts dissolving, a chemical reaction occurs that releases carbon dioxide gas. This is what the bubbles are. Some factors can change how quickly the carbon dioxide gas is made, and consequently affect how furiously the tablet fizzes. In this activity you’ll explore whether you can make an Alka-Seltzer tablet fizz faster or slower by changing the temperature of the water. How does the water’s temperature affect the reaction? The Effect of Temperature - Keegan's Science Lab Procedure: 1. Put 1 Alka Seltzer tablet in 100 mL of cold water and use a stopwatch to record the amount of time it takes for the Alka Seltzer tablet to dissolve. 2. Rinse the beaker with running water 3. Repeat Step 1 and 2 for the 2nd trial. 4. Calculate the mean between the two trials 6. Leave the hot plate on for 1 minute to let it heat up 5. Alka Seltzer Experiment Lab Report - 711 Words | Cram Graph 2 displays the averages of the reaction time which showed the higher the water temperature is, the faster the reaction time would be. At 65⁰C the average time for the Alka-Seltzer to completely dissolve was 1 minute 4 seconds followed by 1 minute 20 seconds at 35⁰C and ending with the highest average at 2 minutes 1 second (1.61).
Alka seltzer temperature experiment. Alka seltzer in different water temperature - Science experiment - YouTube We have lot more kids experiments in this channel. Please visit under "Science Experiments" Playlist. You can find many more kids activities on Instagram. P... DIY At-Home Science Experiments | Alka-Seltzer® Alka-Seltzer® can be used to create some pretty amazing experiments. Some are easy, some advanced. Slap on some safety goggles and let's go. ALL EXPERIMENTS REQUIRE ADULT SUPERVISION. DIY EXPERIMENTS Make potions and rockets and more. (Insert mad scientist laugh here.) Temperature vs. Rate of Reaction See how hot and cold make things hot and cold. Plop, Plop, Fizz Fast: The Effect of Temperature on Reaction Time | Science ... You will fill the drinking glass with the same volume of water at three different temperatures: hot tap water, cold tap water, and ice water. For the hot and cold tap water, run the water until the temperature stabilizes. Fill the glass with water to the level of the masking tape. Be careful when handling the hot water. PDF Alka Seltzer Fizzing—Determination of Percent W by Mass of NaHCO3 in Alka ... in Alka Seltzer tablets can be determined by the method of eudiometry (10). In +the experiment described in this article, an alternative to the work of Peck et al. (10), students analyze the content of NaHCO 3 in an Alka Seltzer tablet by a rather simple method (measuring the weight loss) utilizing a common household material (vinegar).
PDF Introduction to Kinetics Using AlkaSeltzer - chymist.com 1. Weigh two Alka-Seltzer tablets separately. Use the average mass of the tablets for your calculations in this experiment. 2. Measure 100 mL room temperature water into a beaker. Measure the temperature of the water. Add one Alka-Seltzer tablet to the water. Measure the time it takes for the tablet to dissolve. Determine Carbonation Countdown: The Effect of Temperature on Reaction Time ... • Two Alka-Seltzer tablets • Timer or clock that shows seconds • Optional: helper Preparation • Fill one of the jars halfway with ice cubes. Add cold tap water to about an inch from the rim. Stir... PDF LAB: Plop, plop, fizz, fizz Alka Seltzer Lab Objectives: Students will accurately measure the temperature of water (hot & cold) using a thermometer. Students will analyze the data collected and determine if their hypothesis is correct. Students will record class data into a table and correctly graph this data. Students will draw a conclusion, based on collected data. Alka Seltzer Temperature Experiment Results | ipl.org Alka-Seltzer Experiment 853 Words | 4 Pages Introduction Alka-Seltzer has been on the market since 1931 and has helped to relieve indigestion and upset stomach. The tablets began to fizz and bubble when dropped into water. "The fizziness happens when baking soda (sodium bicarbonate) and citric acid react chemically in water.
Alka-Seltzer Experiment - 78 Words | Bartleby In this experiment, the scientists will be analyzing on whether temperature affects the rate of a gas producing reaction. The independent variable would be the temperature of water in degrees Fahrenheit, while the dependent variable is the rate of reaction which is caused by the rate of carbon dioxide produced in minutes. PDF Plop-Plop Fizz-Fizz: How Does Surface Area and Temperature Affect Chemical ... for this project was if the powdered Alka-Seltzer tablet is added to 30 ( water, then the chemical reaction rate will be faster than any other temperature or particle size tested. The constant variables in my experiment were the amount of water and Alka-Seltzer used, and the size of the glass used. PDF The Greenhouse Effect: Does the Concentration of Carbon Dioxide Affect the ... When Alka-Seltzer is dissolved in water, CO 2 is released and carried to the surface by small bubbles. As bubbles reach the surface, CO 2 is released into the air above the water's surface. This equation explains what happens when an Alka-Seltzer tablet is dissolved in water. C 6 H 8 O 7 (aq) + 3NaHCO 3 (aq) → 3H 2 O(l) + 3CO 2 (g) + Na 3 C ... Alka Seltzer & Temperature Experiment - Study.com Dec 22, 2021 · Experiment Methods 1. Fill one cup halfway with water and put it in the fridge for at least 10 minutes. This is your cold water. 2. Fill another cup halfway with water and leave it on the counter...
Hypothesis & Purpose - Alka Seltzer Project Hypothesis: I predict that the temperature affects the dissolving rate of alka seltzer because I think in hot water there would be more energy to dissolve the substance faster than rather in cold water. Purpose: The purpose of this experiment is to measure the effect of temperature on the rate of a chemical reaction. Also I want to find if the rate of dissolving alka seltzer in different ...
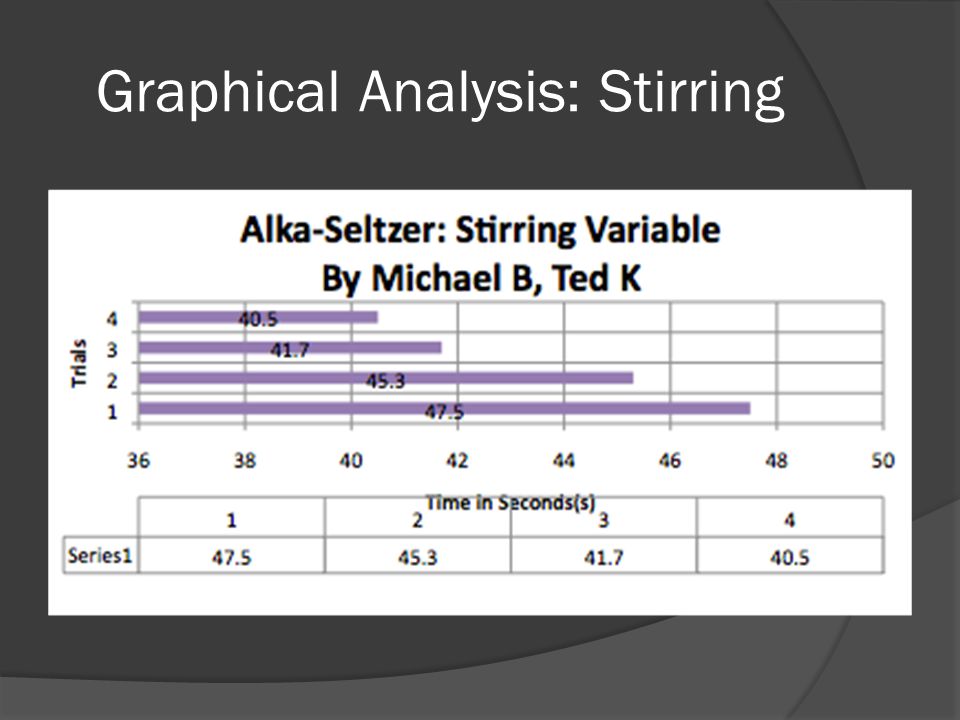
The Alka Seltzer Reaction - Middle School Chemical Engineering For Girls In this activity, students will experiment with the reaction between Alka Seltzer tablets and water in different conditions. By changing temperature and the surface area available for reaction, students will begin to see what factors chemical engineers can control to get the desired result.
Alka Seltzer and Water Chemical Reaction - Video & Lesson Transcript - Study.com Now, we can go through out experiment steps: 1. First, get your water to the right temperature. Take three identical cups and label one hot, one room temperature, and one cold. 2. Start by putting...
Effect of Temperature on Rate of Reaction | Alka-Seltzer® Part A. Hot Water Run water from the hot tap until it is as hot as possible. Fill a clear glass with exactly 8 oz./240 mL of hot water. Use the thermometer to take the temperature and record it on your data sheet. Remove 1 Alka-Seltzer tablet from its package. Drop it into water. Measure the time required for tablet to fully dissolve.
Alka Seltzer Experiment Essay - 1462 Words | Cram My hypothesis for this experiment would be that the Alka Seltzer that dissolves the fastest would be the one that was placed in the water with the highest temperature. There will be controlled and manipulated variables explained throughout the experiment.
PDF Temperature's Effect on Reaction Rates Experiment Procedure 1. Use one piece of tape to attach one alka-seltzer tablet to rounded end of the spoon—be sure the spoon is dry first! 2. Find the temperature of the water using the thermometer—the water
Alka Seltzer Experiment - 179 Words | Bartleby In this experiment, the scientists will be analyzing on whether temperature affects the rate of a gas producing reaction. The independent variable would be the temperature of water in degrees Fahrenheit, while the dependent variable is the rate of reaction which is caused by the rate of carbon dioxide produced in minutes.
Alka Seltzer Experiment - Summarized by Plex.page | Content | Summarization Apr 10, 2020 · Hydrogen ions in water can react with sodium bicarbonate's base inside the Alka Seltzer tablet base. Get your timer and drop the Alka Seltzer tablet in. For the room temperature water and the cold water, repeat step 5 for the room temperature water and the cold water. When heat is added to a substance, the molecules will move faster.
Us the scientific method to solve an experiment with Alka-Seltzer® tablets ... In this exercise, the scientific method is used toinvestigate the rate at which effervescent tablets dissolve in water and to identify thefactors that affect this rate. After an initial experiment, a question will be developed,and the scientific method will be applied to answer it.
Lab Report Alka-Seltzer | PDF | Thermometer | Temperature • 9 alka-seltzer tablets • 2 burners Procedure: 1) Fill your beakers until they are at 200 mL 2) Place one beaker on one burner and the other beaker on the other burner 3) The heat level on one burner should be at level two and the other burner should be level four 4) Place thermometers in each of these beakers to measure the beginning temperatures
Alka Seltzer Experiment Lab Report - 711 Words | Cram Graph 2 displays the averages of the reaction time which showed the higher the water temperature is, the faster the reaction time would be. At 65⁰C the average time for the Alka-Seltzer to completely dissolve was 1 minute 4 seconds followed by 1 minute 20 seconds at 35⁰C and ending with the highest average at 2 minutes 1 second (1.61).
The Effect of Temperature - Keegan's Science Lab Procedure: 1. Put 1 Alka Seltzer tablet in 100 mL of cold water and use a stopwatch to record the amount of time it takes for the Alka Seltzer tablet to dissolve. 2. Rinse the beaker with running water 3. Repeat Step 1 and 2 for the 2nd trial. 4. Calculate the mean between the two trials 6. Leave the hot plate on for 1 minute to let it heat up 5.
Alka-Seltzer Science: The Effect of Temperature on Reaction Time | STEM Activity Have you ever wondered why bubbles form when an Alka-Seltzer tablet is put in water? If you’ve ever tried it, you’ve seen that the tablet fizzes furiously when dropped into water. The moment the tablet starts dissolving, a chemical reaction occurs that releases carbon dioxide gas. This is what the bubbles are. Some factors can change how quickly the carbon dioxide gas is made, and consequently affect how furiously the tablet fizzes. In this activity you’ll explore whether you can make an Alka-Seltzer tablet fizz faster or slower by changing the temperature of the water. How does the water’s temperature affect the reaction?




























0 Response to "43 alka seltzer temperature experiment"
Post a Comment