40 endothermic and exothermic reactions lab
Difference Between Endothermic Reactions and Exothermic ... The endothermic reaction makes the surroundings cool by absorbing energy on the flip side; exothermic reaction makes the surroundings hot by releasing temperature. Energy is absorbed in endothermic reaction while in exothermic reactions, energy is usually released in the form of heat, but can also be in the form of electricity, light or sound. Exothermic and Endothermic Reactions - C-DAC Online Lab Chemical reactions and equations (Click here to read) Exothermic reaction. Endothermic reaction. YouTube. Studynlearn. 71.7K subscribers.
Determination of the Enthalpy of an Acid-Base Reaction ... As such, when holding a container having an exothermic reaction your hand feels warm. The vice versa is true for endothermic reactions. When calculating, a negative value depicts an exothermic reaction while a positive value represents an endothermic reaction. With respect to this experiment, the reaction represents an exothermic reaction.

Endothermic and exothermic reactions lab
Endothermic and Exothermic Reaction Lab - Hamilton Local ... This week you will learn about endothermic and exothermic reactions. BE VERY CAREFUL WITH THE CHEMICALS YOU ARE USING AND PRODUCING. IF YOU.3 pages Wikipedia:Vital articles/List of all articles - Wikipedia 0 (1) 0 1 (102) 1 · 1 Maccabees · 1,1,1,2-Tetrafluoroethane · 1,2-rearrangement · 1,4-Dioxane · 10 · 10 Hygiea · 10,000 metres · 100 metres · 100 metres freestyle · 10th Dalai Lama · 11th Dalai Lama · 12 Angry Men (1957 film) · 120-cell · 12th Dalai Lama · 13th Dalai Lama · 14th Dalai Lama · 1500 metres · 1556 Shaanxi earthquake · 16-bit computing · 16-cell · 16th Street ... Endothermic/Exothermic Lab
Endothermic and exothermic reactions lab. Endothermic And Exothermic Experiment Lab Answers ... Get Free Endothermic And Exothermic Experiment Lab Answers Including The Conclusion in chemical education.Use research- and brain-based teaching to engage students and maximize learning Lessons should be memorable and engaging. When they are, student achievement increases, behavior problems decrease, and teaching and learning are fun! Chemical Reactions (Simulator) : Class 9 - Amrita Online Lab To carry out the following reactions and classify them as physical or chemical changes. a. Iron with copper sulphate solution in water. b. Burning of magnesium in air. c. Zinc with dilute sulphuric acid d. Heating of copper sulphate e. Sodium PDF Memo Grade 11 Investigate Endothermic Reactions Where To Download Memo Grade 11 Investigate Endothermic Reactions Memo Grade 11 Investigate Endothermic Reactions Getting the books memo grade 11 investigate endothermic reactions now is not type of inspiring means. You could not unaccompanied going in the same way as books stock or library or borrowing from your links to contact them. PDF Chemical Reaction Lab Report Grade 10 Lab w/ Link to Lab Report Chemical Reaction Lab Report Grade Exothermic reactions are exactly the opposite. While they take some energy to get going, called the activation energy of reaction, these reactions give off heat during the reaction. Good examples of ... Endothermic and Exothermic Reactions Experiment Search by subject and grade level ...
Virtual Lab Autograded problem: Camping To do this in the Virtual Lab, use the "Thermal Properties" dialogue. Windows users should right click on the beaker or flask; Mac-users should command-click on the beaker or flask. From the menu that appears, choose "Thermal Properties." Check the box labeled "insulated from surroundings." Exothermic and Endothermic Reactions (Endothermic) : Class ... तुम्ही इथे आहात->होम->Biology->Class 9->Exothermic and Endothermic Reactions. Exothermic and Endothermic Reactions. Reaction Exposed: The Big Chill! - Activity - TeachEngineering Students investigate this endothermic reaction. They test a stoichiometric version of the reaction followed by testing various perturbations on the stoichiometric version in which each reactant (citric acid, sodium bicarbonate and water) is strategically doubled or halved to create a matrix of the effect on the reaction. Lesson 1 Exothermic And Endothermic Reactions Exothermic chemical reactions release energy, while endothermic reactions absorb energy. But what causes some reactions to be exothermic, and others to be endothermic? In this simulation, compare the energy absorbed in breaking bonds to the energy released in forming bonds to determine if a reaction will be exothermic or endothermic.
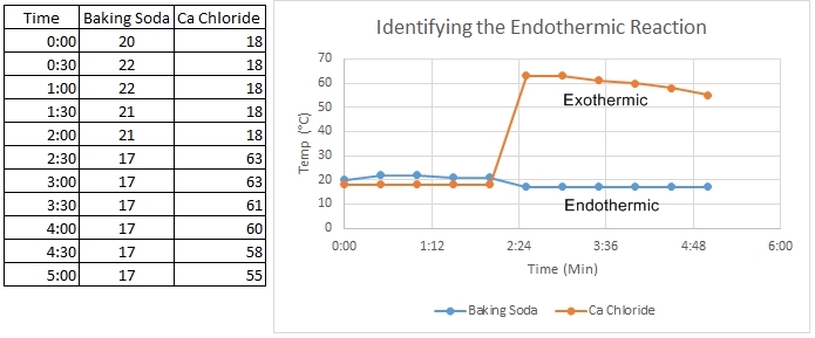
Endothermic and Exothermic Reactions Some chemical reactions absorb energy and are called endothermic reactions. You will study one exothermic and one endothermic reaction in this experiment.5 pages Endothermic And Exothermic Experiment Lab Answers ... Apr 07, 2021 · Download free calorimetry lab gizmo quiz answers … gartenleidenschaft.de Exothermic/Endothermic — Exothermic reactions give off heat-the chemical reaction makes heat. Endothermic reactions lower the temperature of the product. You are going to combine different materials and test to see if the reaction is exothermic or ... PDF Endothermic And Exothermic Experiment Lab Answers ... Endothermic and Exothermic Reactions Lab ? iTeachly.com Exothermic or endothermic? ?H (+/?) Baking soda + vinegar ~22 °C 15 °C ?7 °C Endothermic + Baking soda solution + calcium chloride ~22 °C 42 °C +20 °C Exothermic ? 1. Based on your observations of the baking soda and vinegar reaction, is the reaction exo-thermic or endothermic? Exothermic And Endothermic Reactions In Everyday Life William Murray presents a lab for high school chemistry students on exothermic and endothermic reactions. Murray includes a list of the materials required, the time needed, and the procedures. Teachers.Net provides the lab as part of the Teachers.Net Lesson Exchange online resource.
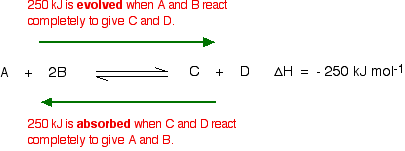
Is exergonic the same as endothermic or exothermic ... ##ΔH## decreases for an exothermic process and increases for an endothermic process. ##ΔG## decreases for an exergonic process and increases for an endergonic process. For a given reaction, the change in Gibbs free energy is ##ΔG## is a measure of the spontaneity of a reaction. If ##ΔG## is negative, the process is spontaneous.
Endothermic & Exothermic Reactions - UGA Extension Some chemical reactions absorb energy and are called endothermic reactions. You will study one exothermic and one endothermic reaction in this experiment.8 pages
PDF Lesson 1 Exothermic And Endothermic Reactions Download Free Lesson 1 Exothermic And Endothermic Reactions 1, 3, 5 and 6. 4.5.1.1 Exothermic and endothermic reactions required ... This website and its content is subject to our Terms and Conditions.
Homework help for students | Chemistry lab » Paper Research Chemistry lab In various chemical processes such as reactions and the dissolving of salts, heat is either absorbed or given off. We call these events either an endothermic (heat in) or exothermic (heat out) process. It is usual to detect these heat events by measuring the temperature change associated with the process.
7. Endothermic reactions absorb a lot of energy ... Chemistry. 7. Endothermic reactions absorb a lot of energy. Exothermic reactions give off a lot of energy. Which type of reaction has a higher activation energy? Which one takes more energy to break up the bonds of the reactants? (1 point) Endothermic reactions have a higher activation energy. Exothermic reactions require more energy to break ...
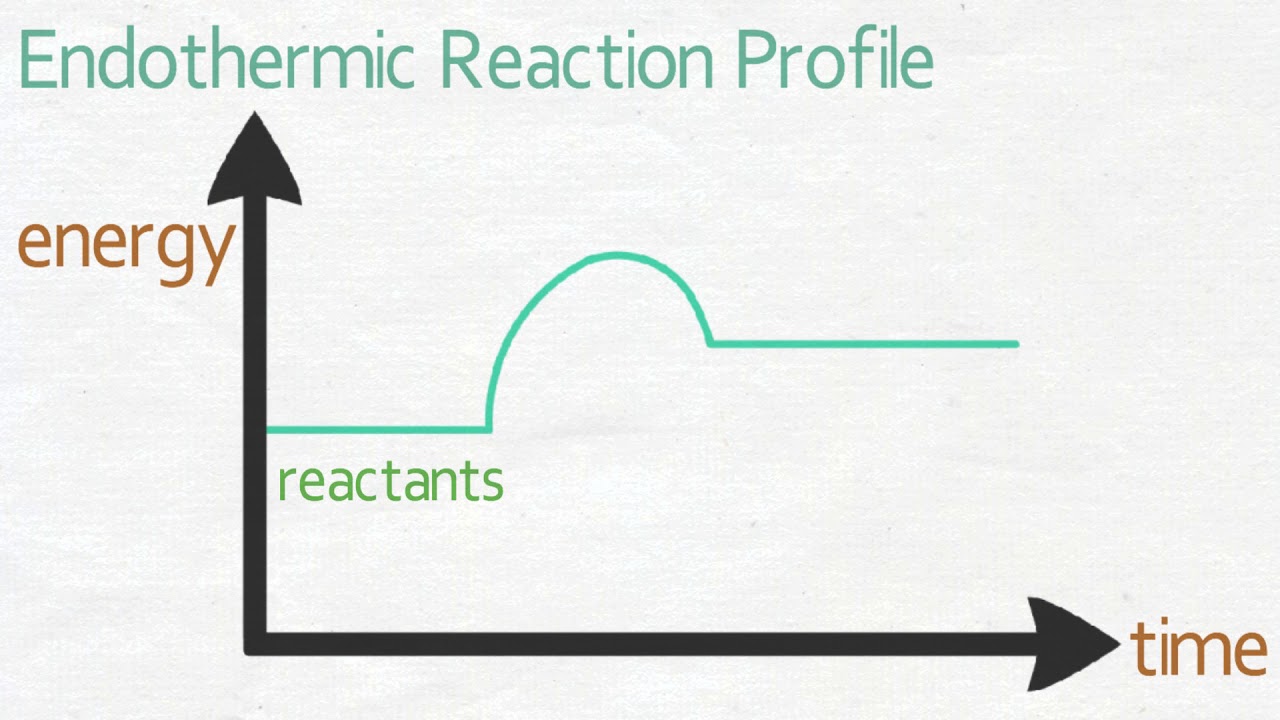
Thermochemistry (Theory) : Class 12 : Chemistry : Amrita ... Thermochemistry is the study of heat and energy associated with a chemical reaction or a physical transformation. A reaction may absorb or release energy. The reaction which absorbs energy in the form of heat is called endothermic reaction and that which releases energy in the form of heat is called exothermic reaction.
Lesson 1 Exothermic And Endothermic Reactions Oct 24, 2013 · The reactions in which energy is released are known as exothermic reactions. In other words, energy is supplied to endothermic reactions and is obtained from exothermic reactions. The given CH4(g) + 2O2(g) -> CO2(g) + 2H2O(g) Why is this 1 / 2 Get Free Lesson 1 Exothermic And Endothermic Reactions autoadvisor.stevens.edu
Applied Physical Science (APS): Chemical Reactions Monday, 5/16: Due: None In Class: Lab Quiz, Notes - Balancing Chemical Equations, Gizmo - Balancing Chemical Equations. HW: Finish Lab Graph and Questions, Study for Test #10. Absent Work: EdPuzzle - Balancing Chemical Reactions. Tuesday, 5/17:
PDF Chemical Reaction Lab Report Grade 10 Reactions. Grade 10 Types of Reactions Pre-Lab w/ Link to Lab Report Chemical Reaction Lab Report Grade Exothermic reactions are exactly the opposite. While they take some energy to get going, called the activation energy of reaction, these reactions give off heat during the reaction. Good examples of ... Endothermic and Exothermic Reactions ...
Endothermic vs. Exothermic Reactions Lab Name: Period: Exothermic Reactions Lab. Name: Period: Objective: The purpose is to investigate Endothermic and Exothermic reactions and to see their effect on the.2 pages
Endothermic and Exothermic Reactions Experiment | Science ... Learn about endothermic and exothermic reactions and energy exchange by experimenting with temperature change in chemical reactions.Acetone *do this one outside!*: Half a Styrofoam ...Liquid: AdditiveHydrogen Peroxide: Dry yeast
Student Lab Guides for Science courses - Edgenuity Lab: Endothermic and Exothermic Reactions: Student Document: Lab: Limiting Reactant and Percent Yield: Student Document: Lab: Rate of Chemical Reactions: Student Document: Lab: Types of Reactions: Student Document
Ward's® Chemistry Exothermic and Endothermic Reaction ... In these demonstrations, exothermic and endothermic reactions are safely carried out inside a plastic bag so students can pass the bag around and feel the results of the reaction. For the exothermic reaction the bag will become warm to the touch, while in the endothermic reaction the bag will become cold to the touch.
PDF Chemical Reaction Lab Report Grade 10 Grade 10 Types of Reactions Pre-Lab w/ Link to Lab Report Chemical Reaction Lab Report Grade Exothermic reactions are exactly the opposite. While they take some energy to get going, called the activation energy of reaction, these reactions give off heat during the reaction. Good examples of ... Endothermic and Exothermic Reactions Experiment
PDF Chemical Reaction Lab Report Grade 10 Types of Chemical Reactions Lab Experiment #3: Types of Chemical Reactions. Grade 10 Types of Reactions Pre-Lab w/ Link to Lab Report Chemical Reaction Lab Report Grade Exothermic reactions are exactly the opposite. While they take some energy to get going, called the activation energy of reaction, these reactions give off heat during the reaction.
What do endothermic and exothermic mean? | Overnight Writers Endothermic means absorbs heat. Exothermic means releases heat. Think of it this way. ##"EX"##othermic means ##"EX"##it. If the heat is ##"EX"##iting, then it is an ##"EX"##othermic reaction. This means that the system goes to a lower energy level. The burning of hydrogen gas is an exothermic reaction.
Endothermic/Exothermic Lab










0 Response to "40 endothermic and exothermic reactions lab"
Post a Comment