40 does sugar ionize in water
› 35755359 › Organic_Chemistry_ByOrganic Chemistry By Clayden Greeves Warren and Wothers Enter the email address you signed up with and we'll email you a reset link. Will the solution of sugar in distilled water conduct electricity? - Byju's Distilled water can ionize H 2 O to H + and OH -, but the degree of ionization far lesser degree to conduct electricity. Adding Sugar C 12 H 22 O 11 to distilled water can also not conduct electricity as sugar does not dissociates into ions. Therefore, sugar solution in distilled water cannot conduct electricity. Suggest Corrections. 0.
alevelchemistry.co.uk › notes › ionic-equationsIonic Equations | A-Level Chemistry Revision Notes - Typical examples are – DI water, ethanol, sugar (C 6 H 12 O 6 – glucose), some other organic compounds, etc. Electrolytic Dissociation. Electrolytic dissociation, also referred to as ionic dissociation, occurs when an electrolyte is dissolved in an aqueous solution (meaning that it is dissociated into ions). Since water is a polar ...
Does sugar ionize in water
Can sugar conduct electricity when dissolved in water? - Quora Sugar does not ionize when dissolved in water, so it does not produce ions that are necessary to conduct electricity. That being said, water undergoes a process called self-ionization in which 2 H2O <=> H3O+ + OH- When H2SO4, sulfuric acid, is added to water, it will ionize into - eNotes Sulfuric acid is considered a strong acid that will ionize completely in water, but this only applies to the first proton. The equation for the first ionization, producing hydrogen sulfate ion, is ... Can a sugar solution conduct electric current? | Socratic This is an exceedingly small amount of ionization. The water conducts so little current that it is often called a nonconductor. If tap water is used to make the sugar solution, the ions in the tap water will increase the conductivity about 500-fold. It is the water in the sugar solution that has the ability to conduct electricity, not the sugar.
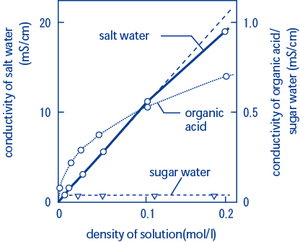
Does sugar ionize in water. PhET: Free online physics, chemistry, biology, earth science and … Founded in 2002 by Nobel Laureate Carl Wieman, the PhET Interactive Simulations project at the University of Colorado Boulder creates free interactive math and science simulations. PhET sims are based on extensive education research and engage students through an intuitive, game-like environment where students learn through exploration and discovery. Will salts ionize in water? Explained by FAQ Blog You can see that at all temperatures, many more grams of sugar dissolve than salt. The graph also shows that the solubility of sugar increases much more than the solubility of salt as the temperature of the water increases. Alum is the least soluble until the temperature of the water increases to about 65 °C. Empty string - Wikipedia The empty string is a syntactically valid representation of zero in positional notation (in any base), which does not contain leading zeros. Since the empty string does not have a standard visual representation outside of formal language theory, the number zero is traditionally represented by a single decimal digit 0 instead. Zero-filled memory area, interpreted as a null … Why Does Water Dissolve Sugar? - Middle School Chemistry
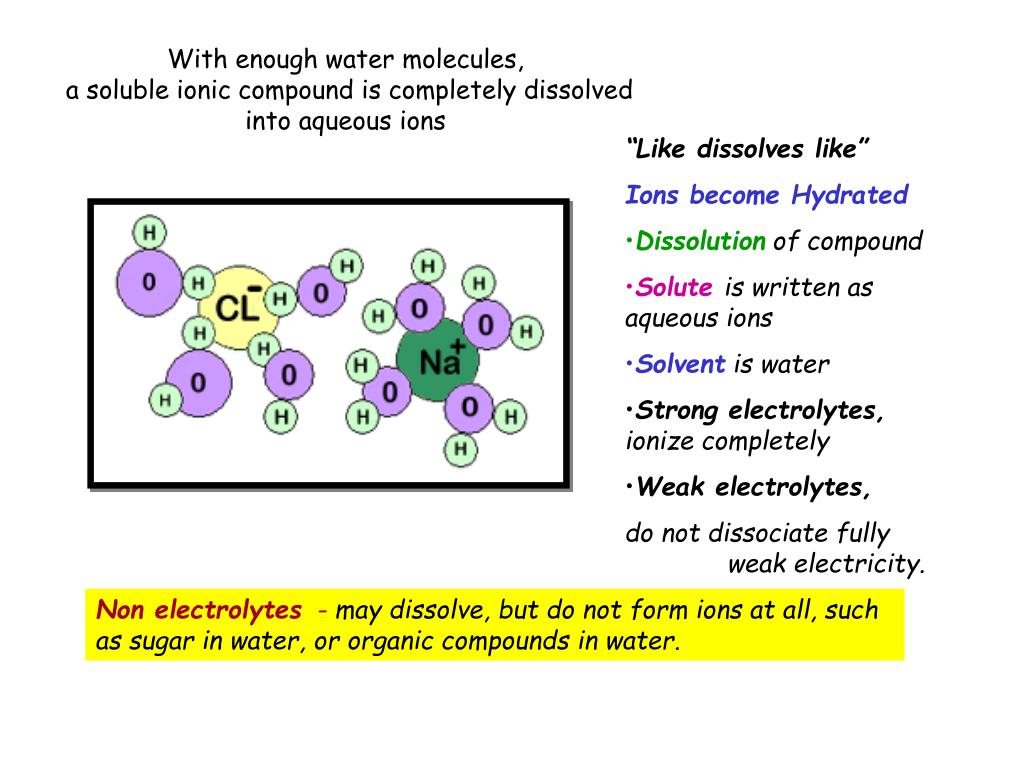
en.wikipedia.org › wiki › Matrix-assisted_laser_deMatrix-assisted laser desorption/ionization - Wikipedia A solution of one of these molecules is made, often in a mixture of highly purified water and an organic solvent such as acetonitrile (ACN) or ethanol. A counter ion source such as trifluoroacetic acid (TFA) is usually added to generate the [M+H] ions. A good example of a matrix-solution would be 20 mg/mL sinapinic acid in ACN:water:TFA (50:50: ... Question: Does sugar dissolve in boiling water? - Let's eat? How much sugar can dissolve in boiling water? A certain amount of water at room temperature can only dissolve up to a certain amount of solute: 1 cup of water can dissolve 1 gram of sugar, 5 grams of sugar, 10, 50, and even 100 grams of sugar; however, 500 grams is too much. 1 cup of water can dissolve a maximum of about 420 grams of sugar. nap.nationalacademies.org › read › 131655 Dimension 3: Disciplinary Core Ideas - Physical Sciences ... A system can appear to be unchanging when processes within the system are occurring at opposite but equal rates (e.g., water behind a dam is at a constant height because water is flowing in at the same rate that water is flowing out). Changes can happen very quickly or very slowly and are sometimes hard to see (e.g., plant growth). Can sugar ionize in water? - Answers Where does sugar go when it is dissolved in water? ... C12H22O11 is sucrose, or table sugar. It does not ionize in solution, and so is a non electrolyte. It will not conduct electricity.
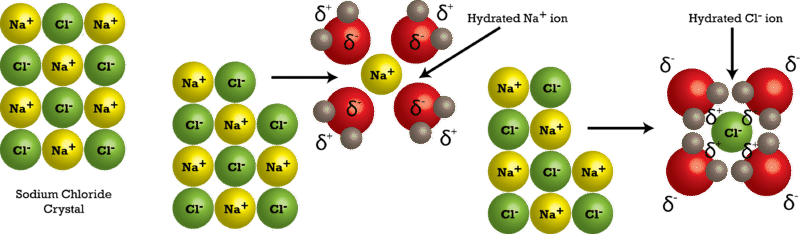
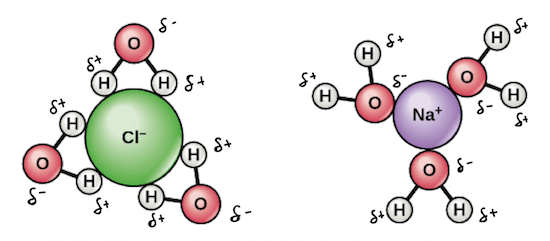
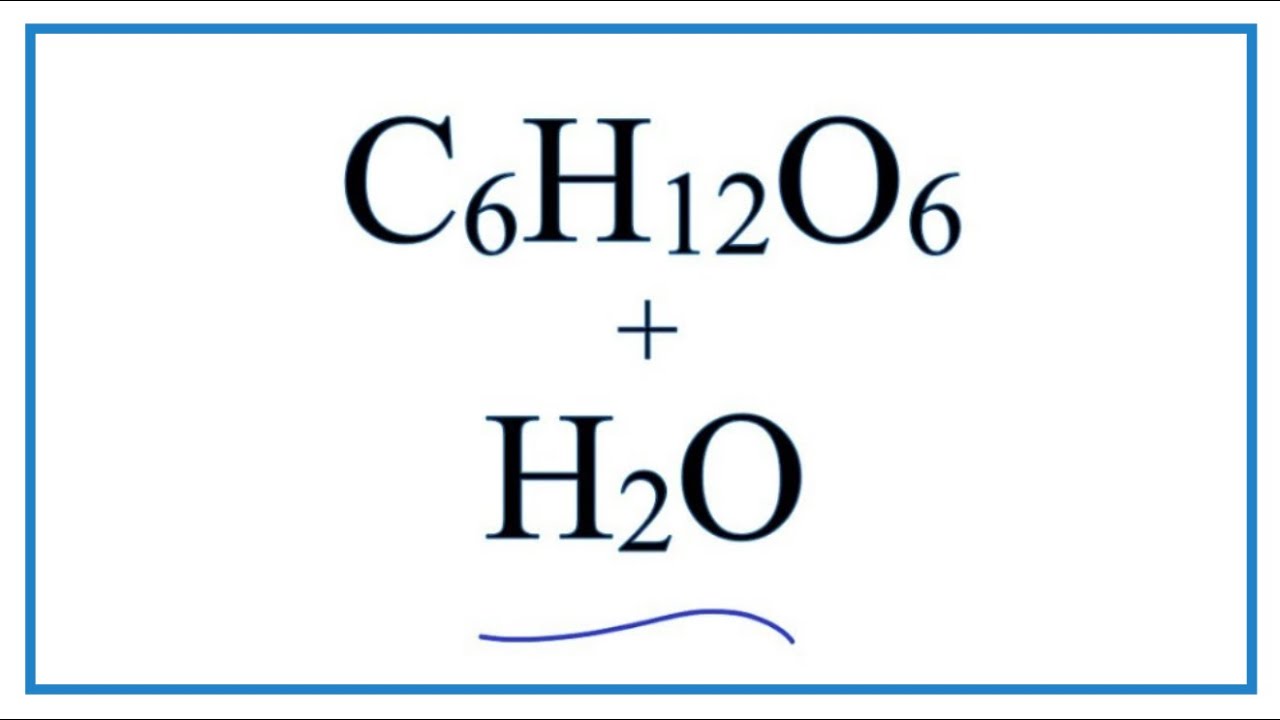
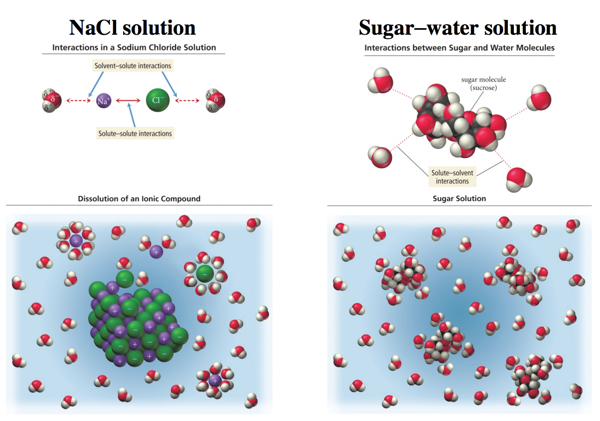
Solubility and Complex-Ion Equilibria - Purdue University In the case of sugar and water, this process works so well that up to 1800 grams of sucrose can dissolve in a liter of water. Ionic solids (or salts) contain positive and negative ions, which are held together by the strong force of attraction between particles with opposite charges. Why does salt solution conduct electricity, while sugar solution doesn't? A nonelectrolyte is a chemical that dissolves in water without producing ions. An aqueous solution of a nonelectrolyte usually doesn't conduct an electric current. Examples of nonelectrolytes include sugar (C 12 H 22 O 11 ), ethanol (CH 3 CH 2 OH), and acetone (CH 3 OCH 3 ). Generally, water soluble molecular compounds are usually non-electrolytes. Does Glucose Form Ions In Water | DiabetesTalk.Net Sugar dissolves inwater because energy is given off when the slightly polar sucrose molecules formintermolecular bonds with the polar water molecules. The weak bonds that form between thesolute and the solvent compensate for the energy needed to disrupt the structure of boththe pure solute and the solvent. How does dissolving a salt molecule in water make its atoms ionize ... The atoms have therefore been ionized by the reaction that forms solid table salt, all without the presence of water. Both the sodium and the chlorine ions now have completely filled shells and are therefore stable. This is a good example of an atom that naturally has an unequal number of electron and protons.
Why do some substances ionize instead of dissolving in water? Finally, glucose readily dissolves in acid, but none of its protons are readily lost so it doesn't ionize at all meaning that it isn't an electrolyte in water.
chem.libretexts.org › Bookshelves › Physical_andRaoult's Law - Chemistry LibreTexts Sep 23, 2022 · In any real solution of, say, a salt in water, there are strong attractions between the water molecules and the ions. That would tend to slow down the loss of water molecules from the surface. However, if the solution is sufficiently dilute, there will be good-sized regions on the surface where you still have water molecules on their own.
Solution Chemistry | Grandinetti Group And example of a non-electrolyte is sugar. Sugar will readily dissolve in water but doesn't form cations and anions in solution. That is, there are no charge carriers formed. ... Substances that only partially ionize into ions when dissolved in water are called weak electrolytes. For example, Acetic Acid (HC 2 H 3 O 2) dissolves in water, but ...
Dissolution of sugar in water and its temperature dependence Since sugar can be dissolved in water, it must be true that the final state is more stable than the initial state, even at elevated temperature. You can do a simple experiment at home to verify this is the case. Using thermodynamics terminologies, Δ G = Δ H − T Δ S, where G is Gibbs free energy, H is enthalpy, S is entropy, T is ...
Is sugar dissolving in water a chemical change? - Nutri Inspector Sugar dissolving in water is not, in fact, a chemical change. Sugar is classified as a simple carbohydrate because it is made up of only one molecule. Carbohydrates are neither acidic nor basic and they do not react with each other. Furthermore, sugar does not react with the water molecules to form new bonds or break old ones.
Hydration May Prevent Blood Sugar Problems - Life Ionizers A new study on hydration shows that drinking water - at least four or more 8 ounce glasses per day - could substantially reduce the chances of developing high blood sugar problems (hyperglycemia). French researchers report. The study followed 3,615 men and women with healthy blood sugar levels for nine years.
Why does salt dissolve in water faster than sugar? - Short-Fact Sugar can dissolve easier than salt because when you add 8 spoons of salt into water the salt already starts to stay in the bottom of the cup. And, when you add 30 spoons into water the sugar just starts to stay in the bottom of the cup. It's almost the same. Why does salt dissolve faster in hot water than cold water?
Dissolving Sugar in Water: Chemical or Physical Change? - ThoughtCo Dissolving sugar in water is an example of a physical change. Here's why: A chemical change produces new chemical products. In order for sugar in water to be a chemical change, something new would need to result. A chemical reaction would have to occur. However, mixing sugar and water simply produces... sugar in water!
Correct 2 sugar does not ionize in gasoline 3 there correct 2 Sugar does not ionize in gasoline 3 There are dipole dipole. Correct 2 sugar does not ionize in gasoline 3 there. School University of Texas; Course Title CH 302; Type. Homework Help. Uploaded By SingATune. Pages 7 Ratings 100% (2) 2 out of 2 people found this document helpful;
Why Does Glucose Not Dissociate In Water | DiabetesTalk.Net Sugar dissolves inwater because energy is given off when the slightly polar sucrose molecules formintermolecular bonds with the polar water molecules. The weak bonds that form between thesolute and the solvent compensate for the energy needed to disrupt the structure of boththe pure solute and the solvent.
› lakhmir-singh-chemistry-class-10Lakhmir Singh Chemistry Class 10 Solutions Acids,Bases And Salts (c) Distilled water does not conduct electricity because it does not contain any ionic compounds dissolved in it whereas rain water does. Reason: When rain water falls on earth through the atmosphere, it dissolves an acidic gas ‘carbon dioxide’ from the air and forms carbonic acid (H 2 CO 3). The carbonic acid provides some hydrogen and ...
If a sugar is nonreducing, does that mean it doesn't ionize in water? Actually, in the case of a reducing sugar, it donates an electron. When it does so, it is oxidised. But that doesn't mean that it ionizes in water! When you keep a reducing sugar in the presence of an oxidising agent, the electron is transferred over to the agent, reducing it.
openstax.org › books › anatomy-and-physiology-2e2.4 Inorganic Compounds Essential to Human Functioning - OpenStax The ratio of sugar to water in the left side of the glass would be the same as the ratio of sugar to water in the right side of the glass. If you were to add more sugar, the ratio of sugar to water would change, but the distribution—provided you had stirred well—would still be even. Water is considered the “universal solvent” and it is ...
On dissolving sugar in water, which ions are formed? - Quora When sugar (sucrose) dissolves in water, no ions are formed. Sugar molecules are distributed in water. Each sugar molecule contains 8 hydroxyl groups, and these groups are stabilized by hydrogen-bonding with water molecules. This is how sugar solution exists. No chemical changes occur when sugar is dissolved in water. Also, no ions are formed.
PDF Sugar or Salt? Ionic and Covalent Bonds - Union University Clean-up: The sugar and salt solutions can be poured down the drain. Rinse the beaker, screw, nail, and stirring rod several times with deionized water. Clean the test tubes with water first and then rinse them with deionized water. They may need to soak for a few minutes in hot water in order to remove the melted substances. 11
Can a sugar solution conduct electric current? | Socratic This is an exceedingly small amount of ionization. The water conducts so little current that it is often called a nonconductor. If tap water is used to make the sugar solution, the ions in the tap water will increase the conductivity about 500-fold. It is the water in the sugar solution that has the ability to conduct electricity, not the sugar.
When H2SO4, sulfuric acid, is added to water, it will ionize into - eNotes Sulfuric acid is considered a strong acid that will ionize completely in water, but this only applies to the first proton. The equation for the first ionization, producing hydrogen sulfate ion, is ...
Can sugar conduct electricity when dissolved in water? - Quora Sugar does not ionize when dissolved in water, so it does not produce ions that are necessary to conduct electricity. That being said, water undergoes a process called self-ionization in which 2 H2O <=> H3O+ + OH-






















:max_bytes(150000):strip_icc()/tablet-in-glass-of-water-521340318-578e32185f9b584d20edefa4.jpg)


0 Response to "40 does sugar ionize in water"
Post a Comment