40 why does alka seltzer dissolve faster in water
Does alka seltzer dissolve faster in hot or cold water? See answer (1) Best Answer. Copy. Alka Seltzer usually dissolves faster in hot water (especially boiling water); it is much slower if you want to dissolve it in cold water. This is because hot ... What type of chemical reaction is Alka-Seltzer and water? As an Alka-Seltzer tablet dissolves in water, it liberates carbon dioxide. The carbon dioxide dissolves in the water and then comes out of solution as a gas. This carbon dioxide gas has mass, but since it is a gas it escapes from the container and diffuses into the atmosphere. Why does Alka-Seltzer work so fast? Alka Seltzer tablets easily ...
Why does Alka Seltzer fizz? | HowStuffWorks Why does Alka Seltzer fizz? Alka-Seltzer contains citric acid and sodium bicarbonate which is baking soda; when it reacts with water, it produces the fizz. Steven Errico / Getty Images. The fizzing you see when you drop an Alka-Seltzer tablet in water is the same sort of fizzing that you see from baking powder.
Why does alka seltzer dissolve faster in water
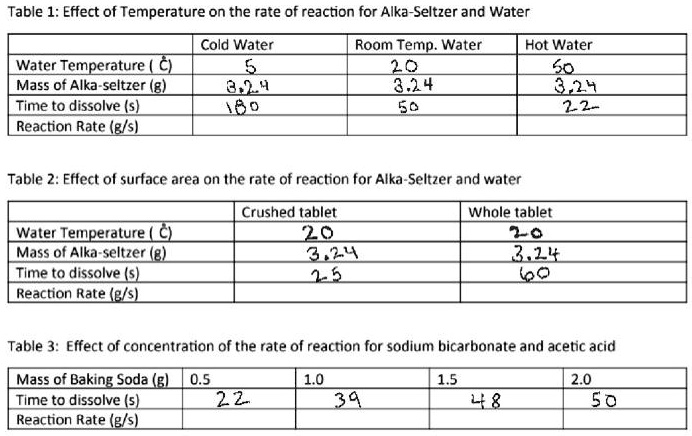
Why does Alka-Seltzer dissolve faster in hot water or cold water? Does Alka-Seltzer dissolve faster in saltwater? We said Alka Seltzer will dissolve fastest in acidic water and slowest in salt water. Our data proves that the Alka seltzer tablet in the warm water dissolves the fastest (24.35 seconds). Our data also proves that the Alka seltzer tablet in the salt water dissolved the slowest (42.76 seconds). Carbonation Countdown: The Effect of Temperature of Reaction Time • Extra: Test Alka-Seltzer tablets in a wider range of temperatures, and then draw a graph showing the time it takes a tablet to dissolve in water at each temperature (check with a thermometer). Does Alka-Seltzer dissolve faster in hot or cold water? The fizz produced when an Alka-Seltzer tablet is dissolved in water is due to the reaction between sodium bicarbonate and citric acid. 3NaHCO3 (aq) + H3C6H5O7 (aq) arrow 3CO2 (g) + 3H2O (l) + Na3C6H5O7 (aq) In a certain experiment, 0.500 moles of sodium bicarb. Using a closed system experiment, will a leak during gas evolving reaction overstate ...
Why does alka seltzer dissolve faster in water. Why does Alka-Seltzer dissolve faster in cold water? Why does Alka-Seltzer dissolve faster in cold water? As the tablets dissolve, the sodium bicarbonate splits apart to form sodium and bicarbonate ions. The probability of the bicarbonate and hydrogen ions doing this is affected by temperature: the higher the temperature, the faster the molecules move; the lower the temperature, the slower they move. Why do Alka-Seltzer tablets dissolve faster in water? - Heimduo Why does Alka-Seltzer tablets dissolve slower in cold water? As the tablets dissolve, the sodium bicarbonate splits apart to form sodium and bicarbonate ions. The probability of the bicarbonate and hydrogen ions doing this is affected by temperature: the higher the temperature, the faster the molecules move; the lower the temperature, the ... why does alka-seltzer dissolve faster in hot than cold water Does Alka-Seltzer dissolve faster in hot or cold water? background: give information about why this lab is important, what key principles are involved and explain them. i need 2 paragrpahs so i was thinking on writing a bit on what alka seltzer is but idk The effect of vinegar concentration on the dissolving rate of Alka-Seltzer The 50% concentration made the liquid become very cloudy, and had less bubbles than the more concentrated solutions.The 25% concentration had the least bubbles/foam, and the tablet dissolved very quickly. Based on the data gathered, the less concentrated the vinegar solution is, the faster the Alka-Seltzer tablet dissolves.
Alka-Seltzer Science: The Effect of Temperature on Reaction Time Alka-Seltzer is a medical drug that works as a pain reliever and an antacid (antacids help neutralize stomach acidity, such as heartburn). The pain reliever used is aspirin and the antacid used is baking soda, or sodium bicarbonate. To take the tablets, they're fully dissolved in water, where they famously undergo a chemical reaction that ... Alka Seltzer and Water Chemical Reaction - Study.com Next, add one cup of water to the cold cup and place it in the fridge. Wait 30 minutes for your cold water to get cold. 4. Now, get your timer ready. Add the Alka-Seltzer tablet to the cold water ... PDF Hannah P. PIngol J0613 - California Science and Engineering Fair The objective is to determine whether the particle size of Alka-Seltzer tablets and the temperature of water affect the reaction time, or the time it takes for the tablets to dissolve in the water. Methods/Materials Alka-Seltzer tablets were used as a particle, and water as a solvent. 12 Alka-Seltzer tablets were used for What factors will make an Alka-Seltzer tablet dissolve faster? The Alka-Seltzer tablet dissolved the quickest in the water because water is the liquid that the Alka-Seltzer tablet was designed to dissolve in. Also, Alka-Seltzer tablets dissolve quicker in larger amounts of liquid compared to smaller amounts of liquid. Why does a tablet dissolve faster in hot water?
Alka-Seltzer Ingredients & FAQs | Alka-Seltzer® Yes, Alka-Seltzer effervescent tablets contain sodium in the form of sodium bicarbonate. The amount of sodium per tablet in each product is listed below. The sodium in the Alka-Seltzer Effervescent products plays an important role, enabling the medication to dissolve quickly in water and begin neutralizing acid as soon as ingested. The Alka Seltzer Reaction - Middle School Chemical Engineering For Girls The chances of this happening are better when the tablet is crushed into more pieces since the molecules have more opportunities to collide and when the temperature is higher, since the molecules are moving faster. In this activity, students will experiment with the reaction between Alka Seltzer tablets and water in different conditions. Why do crushed tablets dissolve faster? - KnowledgeBurrow.com Why does Alka-Seltzer dissolve faster in cold water? As the tablets dissolve, the sodium bicarbonate splits apart to form sodium and bicarbonate ions. The probability of the bicarbonate and hydrogen ions doing this is affected by temperature: the higher the temperature, the faster the molecules move; the lower the temperature, the slower they move. Plop, Plop, Fizz Fast: The Effect of Temperature on Reaction Time Alka-Seltzer is a medical drug that works as a pain reliever and an antacid (antacids help neutralize stomach acidity, such as heartburn). The pain reliever used is aspirin and the antacid used is baking soda (sodium bicarbonate, NaHCO3). To take the tablets, they should be fully dissolved in a glass of water.
Did the Alka-Seltzer dissolve more quickly in hot water? Why does Alka-Seltzer dissolve faster in water than vinegar? As the vinegar concentrated decreased, the water concentration increased. Therefore, the Alka-Seltzer tablet dissolves faster in water than vinegar. When more water was added, there were gradually less bubbles and it took quicker for them to dissipate and the solution to clear.
What does Alka-Seltzer do to water? - KnowledgeBurrow.com As an Alka-Seltzer tablet dissolves in water, it liberates carbon dioxide. The carbon dioxide dissolves in the water and then comes out of solution as a gas. This carbon dioxide gas has mass, but since it is a gas it escapes from the container and diffuses into the atmosphere.
Does Alka-Seltzer dissolve faster in hot or cold water? The fizz produced when an Alka-Seltzer tablet is dissolved in water is due to the reaction between sodium bicarbonate and citric acid. 3NaHCO3 (aq) + H3C6H5O7 (aq) arrow 3CO2 (g) + 3H2O (l) + Na3C6H5O7 (aq) In a certain experiment, 0.500 moles of sodium bicarb. Using a closed system experiment, will a leak during gas evolving reaction overstate ...
Carbonation Countdown: The Effect of Temperature of Reaction Time • Extra: Test Alka-Seltzer tablets in a wider range of temperatures, and then draw a graph showing the time it takes a tablet to dissolve in water at each temperature (check with a thermometer).
Why does Alka-Seltzer dissolve faster in hot water or cold water? Does Alka-Seltzer dissolve faster in saltwater? We said Alka Seltzer will dissolve fastest in acidic water and slowest in salt water. Our data proves that the Alka seltzer tablet in the warm water dissolves the fastest (24.35 seconds). Our data also proves that the Alka seltzer tablet in the salt water dissolved the slowest (42.76 seconds).
































0 Response to "40 why does alka seltzer dissolve faster in water"
Post a Comment